Julius No came up tough. Abandoned by his parents, he fell in with criminals—and eventually it caught up with him. One night, an assassin aimed carefully at his chest, just left of center, and shot him through the heart. Or so he thought. But Julius didn’t die. He went on to medical school, and became an early nemesis of a certain spy named James Bond. Dr. No is a fascinating character, but what surely escapes most fans of Ian Fleming’s novels is that the villain’s miraculous survival reveals a facet of human anatomy that usually remains hidden—a radical asymmetry of left and right.
The difference between our front and back is so obvious that we rarely think about it; we simply don’t have eyes in the back of our head. Top and bottom are obvious, too. Inside, though, asymmetry is everywhere. Heart and stomach lie to the left, the liver to the right. Even our lungs aren’t truly paired; the right lung has three lobes, the left two. And anyone who’s had appendicitis can tell you about the chirality of our guts. The searing abdominal pain radiates from the lower right, not the left.
It’s a story of sperm that can’t swim, a young cardiology fellow, and Japanese street food.
Dr. No survived because his assassin quite reasonably assumed the heart would lie to the left, but his victim was among the very tiny fraction of people who are born with their organs reversed, a mirror image called situs inversus. And while Dr. No is fictional, situs inversus did save the life of at least one real-world gunshot victim, described in a 2020 case study titled, “Shot in the Chest, Saved by the Heart.”
How our organs take on these asymmetrical positions is among the most fascinating questions in developmental biology, the science of embryos. The single cell of the fertilized egg divides again and again, becoming trillions, and those cells literally sculpt our bodies. The process by which this happens is almost implausibly baroque. I am a molecular biologist, and in my 30 years of studying embryos, I’ve found few things quite so fantastical as the tortuous route embryos take to separate right from left.
It’s fitting, then, that the story of how we came to understand these mysteries is equally tortuous. It’s not simply a story of developmental biology, but one of chronic coughing, sperm that can’t swim, a young cardiology fellow, and Japanese street food. Oddly enough, the story opens with another spy, and another doctor named Julius.
Unlike Mr. Bond, Privy Councillor Karl Ferdinand Zivert hadn’t a trace of cloak and dagger panache. It was the decidedly glamourless craft of reading other people’s mail that made him a favorite spy of the last emperor of Russia, Tsar Nicholas II. It also made Zivert rich enough to send a son to medical school, and in 1902, that son published a case report in Russkiy Vrach (The Russian Physician). In it, Alfons-Ferdinand Julius Zivert described just a single patient, a 21-year-old man who’d been coughing violently for his entire life.
The scene in the clinic will sound familiar. The doctor got out his stethoscope, placed it here and there, listened. Then he laid his hands on the patient’s chest and abdomen, pressing here and there, pausing thoughtfully. The routine seems perfunctory, but its remarkable diagnostic power has kept it a staple of medicine for 200 years. It also offers the rare opportunity to appreciate your own left and right.
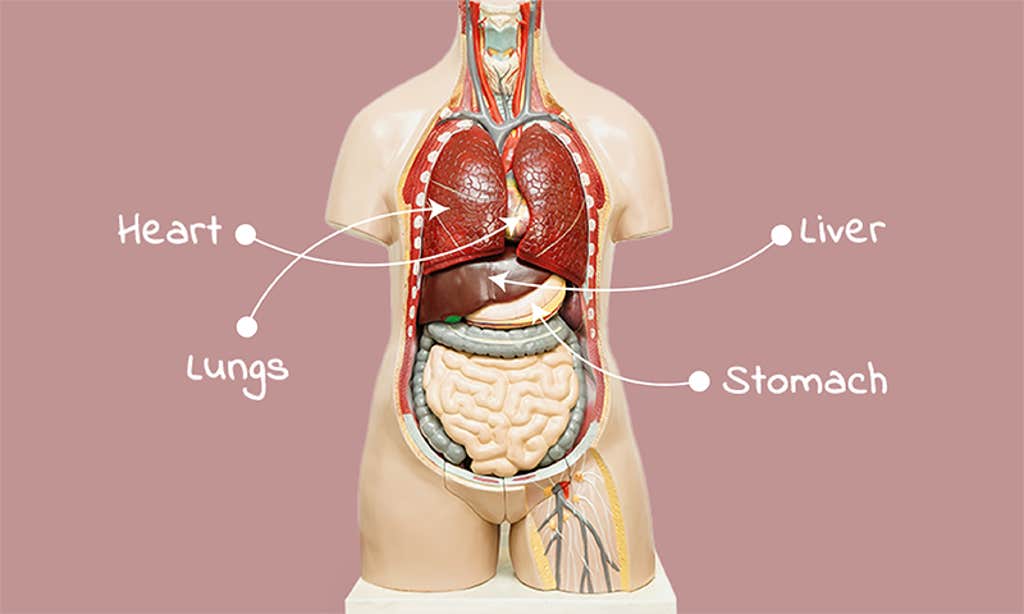
Zivert hoped the sounds in his stethoscope would give up some hint to the nature of that persistent cough. But he didn’t hear what he expected; the lung sounds weren’t even in the right place. Nor were the heart sounds. Pressing gently, he felt for the liver; it too wasn’t where it should be. He listened again, finding the bowel sounds out of place as well. Right there in Kiev, decades before the advent of X-rays, it was entirely obvious that this patient had been born not just with a cough, but also with situs inversus.
Zivert wasn’t the first to observe the condition, so while situs inversus was interesting, it was the cough that fascinated him. Severe and constant, the patient had suffered violent spasms every day since his birth. Whatever was wrong with his lungs, Zivert was certain it had occurred during embryonic development, right alongside the reversal of his organs. In arguing that both the patient’s compromised lung function and left/right asymmetry arose during embryonic development, Zivert took a big step. To make sense of what happened next, though, we need first to consider something that happened long before.
In the late 17th century, Antonie van Leeuwenhoek’s innovations in microscopy made him the first to see what we now know is an entire world of microscopic organisms.
He had discovered impossibly small creatures in pond water and seawater, but the Dutch fabric merchant was aware of his outsider, even amateur, status. So, he hesitated before exploring another fluid. Did anyone really want him to look that closely at his own semen? Well, yes, actually, he decided, they did. And why stop there? He examined the semen of rabbits and dogs. He discovered there a new creature, one with a rounded head and a long tail that beat rhythmically. It swam!
Fearing the findings were obscene, he described them in Latin to lend a veneer of respectability. In 1677, he sent them to the president of the Royal Society of London, advising him “to publish or destroy them, as your Lordship sees fit.” His Lordship did publish them, and that is how the world learned about sperm.
But sperm weren’t the only tails van Leeuwenhoek saw wagging in his new microscopes. Other creatures too were “furnisht with similar instruments in order to make a stir in the water”—tiny aquatic animals studded with hair-like tails called cilia. In the early 19th century, we learned that those creatures with wagging tails includes us. The inside of our lungs and trachea are lined by millions of these cilia. And while they pulse just like sperm tails, the outcome is the opposite. Sperm tails propel the cell through a fluid, but airway cells stay put; cilia propel the fluid. What fluid, you ask? Mucus.
You’ve probably thought about mucus, at least when you’re sick, but while I think about cilia pretty much every day (it’s my job), you might never think of them at all. And yet, mucus and cilia form an inseparable partnership. Mucus is constantly produced in a thin layer, and the cilia beat without pause to move it to your pharynx, where you swallow it. All day and night, you are swallowing mucus, and this slimy treadmill is essential to the health of your lungs. It captures and removes inhaled particles, bacteria, and viruses. When your cilia don’t beat, mucus can’t move; it gets stuck in your lungs, and you cough. Worse yet, you can’t clear those pathogens, so you pretty much always have a cold, or worse, pneumonia. Just like Zivert’s patient in Kiev.
Young Zivert knew about the cilia in our lungs, but he hadn’t an inkling of how they relate to situs inversus. For decades, neither did anyone else, even as X-rays revealed a whole series of similar patients and forged an irrefutable link between situs inversus and lung disease. Then one day in Stockholm, medical scientist Bjorn Afzelius was asked to help figure out why some men’s sperm don’t swim.
Afzelius was a pioneer of the electron microscope, and like van Leeuwenhoek before him, he drew back the veil on a whole new world of sperm, seeing inside them for the first time. In 1959, Afzelius discovered repeated units inside their waving tails that he called “arms.” Within a few years, the same arms were found in all kinds of cilia, from van Leeuwenhoek’s tiny aquatic animals to those in our own airway, a remarkable testament to evolution at work. Today, we know these arms are composed of a molecular motor protein called dynein. Arrayed along the entire length of cilia, they drive the wagging of these extraordinary tails.
It was only natural that Afzelius was asked to examine the sperm of infertile men, for in many patients, sperm are present and look normal, but their tails don’t beat. Maybe some defect could be discerned in the electron microscope? Sure enough, his patients’ sperm lacked dynein arms.
It was a satisfying discovery, but it wouldn’t have made him famous but for the fact that three of the infertile men just kept coughing. Afzelius examined biopsies of their airway cilia, and these too lacked dynein arms. Inevitably, their coughing led to chest X-rays, and those in turn revealed that all three also had situs inversus. Afzelius knew this reversal was rare; it couldn’t be a coincidence. The waving of cilia must somehow be connected to the left/right asymmetry of the body.
Sperm weren’t the only tails van Leeuwenhoek saw wagging in his new microscopes.
Embryos develop progressively, starting simply and becoming more complex bit by bit, so the next big question was where and when in the embryo these still-hypothetical cilia did their thing. Afzelius found a clue in the library when he learned that just across the Baltic Sea in Rostock, Germany—but decades earlier—Kurt Pressler had been tinkering with toads. A practitioner of what is now called “cut and paste” embryology, he used watchmaker’s forceps and fine glass needles to dissect out bits of embryos and reimplant them to see how development might be affected. Afzelius was keen on a report from 1911, in which Pressler described the result of a surgery performed surprisingly early in embryogenesis, not just before organs become asymmetric, but before organs even become.
Embryos lay out their principal axes in a process called gastrulation, at the end of which an expert can discern front from back, head from tail. But at this early point, the embryo still has no organs; left and right exist only as geometry. Nonetheless, Pressler found that when a small part of the back was removed at this stage, rotated 180 degrees, and re-implanted, then some weeks later the organs developed with complete situs inversus.
Pressler said nothing of cilia, but Afzelius sensed the flow of evidence perfectly. Today, we know that motile cilia on that rotated bit of an early frog embryo do control left and right. Cilia in the equivalent region of early mammalian embryos, including humans, do the same. But Afzelius couldn’t prove it, and he complained that he couldn’t test his idea with “a more specific interference.”
Ironically, the very “interference” that ultimately proved Afzelius right had already been discovered, 15 years before his lament. He just didn’t know about it. This was long before the internet, and anyway, it hadn’t come from his colleagues studying male fertility, or from electron microscopists, or even from developmental biologists. No, that discovery came from an expert in breast cancer, a mouse geneticist in Bar Harbor, Maine.
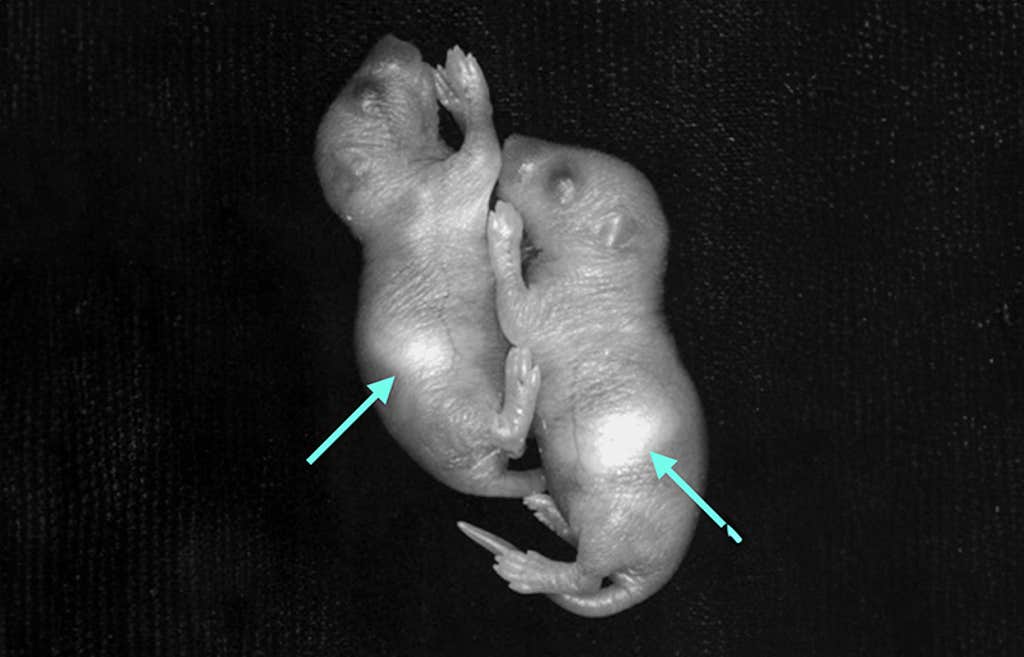
Katherine Hummel was never particularly interested in situs inversus. When the double helix structure of DNA was discovered in the 1950s, she was well known for her studies of the genetic basis of cancer. Cancer runs in the family, not just in humans but in mice as well. Hummel had found such mouse families, and by reliably developing tumors, they allowed her to test treatments, to glean insights. And, in breeding generation upon generation of mice, she inevitably found new families, with new traits. Families of mice with diabetes, families prone to obesity. One day, she observed a litter of suckling pups, their nearly transparent newborn skin revealing tiny white stomachs filled with milk.
To her surprise, two of the pups had that white spot on the right, not the left. She bred the mice further and found that situs inversus was a genetic trait. It ran in the family. She also found that only a single gene was responsible, but with the revolution of molecular biology still decades away, she’d no idea which gene. Just the same, she dutifully published a paper describing the mice in 1959, the very year Afzelius had first found arms in cilia. Hummel never studied the mice again, though others did, and like some rodent royal bloodline, the family bred true for dozens of generations.
Inspiration arrived from two unlikely sources: street food and the oil industry.
In the 1990s, a new breed of “molecular geneticists” started to link such families of mice to specific genes. One of these was Martina Brueckner. Born the same year Hummel published her paper, Brueckner used the descendants of those two pups to add the next piece to Zivert and Afzelius’ growing puzzle.
The mouse genome has nearly 3 billion individual units, and Brueckner found the tiniest possible change. In the ATCG language of genes, changing a single G to an A in the DNA was enough to cripple the dynein motor in the arms; cilia in these mice cannot beat. Even more excitingly, she found that the gene is active specifically in a region of the early mouse embryo called the node, the anatomical equivalent of that rotated bit in Pressler’s toads.
Shigenori Nonaka read Brueckner’s paper closely. Half a world away in Japan, he was using video microscopy to make movies of this very region of the mouse embryo, the node. He saw cilia beating there, but more importantly, he was able to add tiny beads to the fluid around the embryo and track their motion. Like mucus marching toward the pharynx, the beads revealed a striking, robust flow across the node. And it was always from right to left. When he did the same with the mice with situs inversus, the beads didn’t move at all. But while it’s obvious enough how infertility results if sperm can’t swim, or that mucus accumulates if airway cilia don’t beat, it wasn’t at all obvious why or how flow of fluid across the node could cause left/right asymmetry. So, seeing the flow just wasn’t enough; Nonaka remembers his mentor insisting he find a way to change it.
Nonaka set aside the modern tools of molecular genetics and took up the century-old tools of cut-and-paste embryology. Using forceps and fine needles that Pressler would recognize, he carefully dissected early mouse embryos out of the uterus and away from the placenta. Just handling these tiny embryos, perhaps half a millimeter long, is hard enough. But getting them to stay still? And creating an equally tiny flow of fluid across them? It seemed impossible.
Inspiration arrived from two unlikely sources: Japanese street food and the oil industry. Takoyaki is a fried octopus treat popular near Osaka. To position his embryos, Nonaka “trapped” them using tiny closed-end chambers he crafted to resemble traditional octopus traps. The embryos were held in place with the ciliated node waving in the fluid outside. Then, after seeing a picture of devices that smooth the flow of oil through refineries, he fashioned a similar but miniscule pump to generate a smooth but tiny “stir in the water,” to borrow van Leeuwenhoek’s phrase from centuries before.
The contraption worked. Nonaka could precisely control fluid flow across the mouse node. When he drove it leftward, all of the embryos developed normally. When he reversed the flow, they developed situs inversus. Leftward flow, controlled by normal cilia with healthy arms, he reasoned, causes cells on the left side to turn on developmental genes that remain silent on the right.
For Dr. No, situs inversus was a blessing, but for most people it simply is: It makes little impact on a person’s health. One woman even became a minor celebrity, posthumously at least, when an autopsy found she’d lived 99 years without ever knowing her organs were reversed. It’s easy to imagine how. A British car with the steering wheel on the right seems strange to Americans, but it drives just fine. So, too, with our organs. Even flipped, if they are connected in the right way, their physiology is normal.
But there is a dark side to our left and right. Imagine a partial reversal, for example where the heart is flipped but the lungs and liver are not. How could the veins and arteries connecting them find their way in the embryo to the right part of each organ? The fact is, they often don’t. Situs inversus has long been linked to a risk for birth defects, especially those affecting the heart. Hummel noted them in her mice; Pressler’s toads had them, too.
Martina Brueckner knows this dark side all too well. She discovered that 1-in-3-billion genetic anomaly, but she didn’t set out to be a geneticist. She’s a pediatric cardiologist, and the kids in her ICU at Yale Medical School are often very, very sick.
When she was still a fellow, mice with situs inversus seemed a reasonable topic for a short, mandatory research project. But when she first set foot in a mouse colony, she saw those tiny milk-filled stomachs on the wrong side and was hooked. She didn’t stop with finding the gene in Hummel’s mice; she’s spent the last 25 years looking for and finding genes related to our own left and right. Sadly, these discoveries usually come from kids with heart defects.
And this work is important, because partial reversal of the organs (called heterotaxy) is frequently lethal. Indeed, birth defects are the number one cause of death for babies in the United States, and more than twice as lethal as pediatric cancer for children of all ages. Heart defects are the most common among them, and while some are treatable with surgery, others are incurable.
Now, a host of studies launched by Brueckner, Nonaka, and others working with animal models is enabling us to find genetic variants—subtle ATCG changes like the one in Hummel’s mice—that cause heterotaxy in humans. With advances in human genomics and preimplantation genetic diagnosis, we’re starting to see a path forward to preventing not just congenital heart defects, but perhaps even the lifelong cough that Zivert’s patient suffered.
Back in Dr. No’s underwater lair, James Bond pours a third martini from the shaker. His host with the inverted anatomy parts his thin lips and recounts the aftermath of his brush with death. “I hid myself in the academic world,” he explains, “the world of libraries and laboratories. And there, Mr. Bond, I lost myself in the study of the human body and human mind. Why? Because I wished to know what this clay is capable of. I had to learn what my tools were before I put them to use.”
Absent the villainy, Zivert’s goals were the same. So, I wonder about him, and how he returned home that April day after seeing his coughing patient. He must have been puzzled. But could he imagine the convoluted epic of real-life libraries and laboratories that would follow? How slowly we’d learn what the clay is capable of?
Over dinner, James Bond considered his nemesis: “The incredible biography rang true. Not a word of it impossible.” Biology follows a similar script, one that’s incredible but true: What looks like no more than the slightest wiggle in an embryo commands the whole of an invisible axis inside the body and governs the function of the sperm, the lungs, and the heart. ![]()
Lead image: Malchev / Shutterstock






