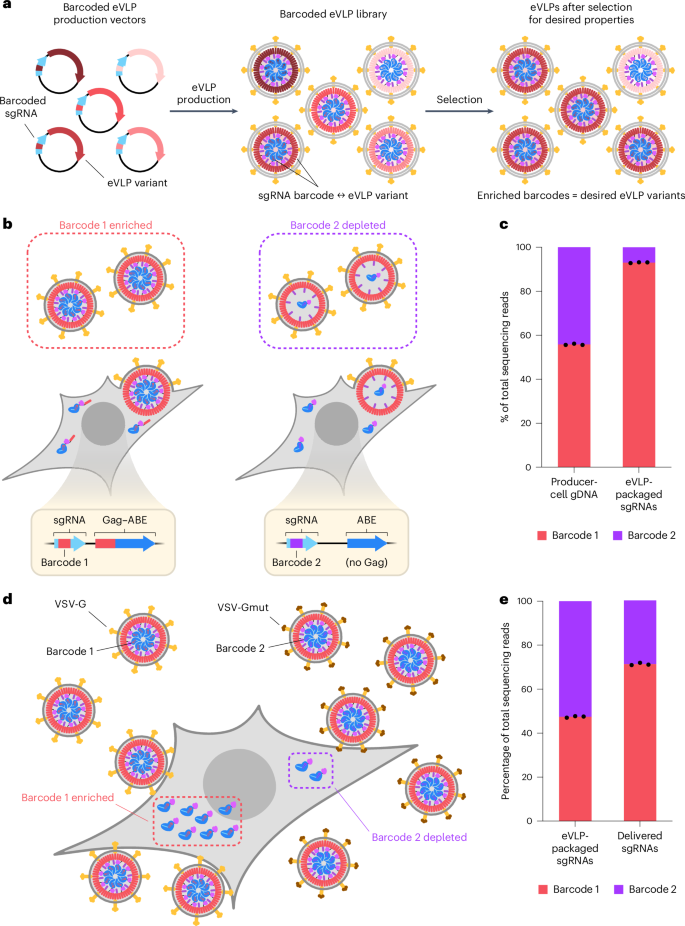
Barcoded guide RNAs identify eVLPs with distinct properties
All directed evolution systems require a way to identify desired variants following a selection for specific properties47,48. To overcome the challenge created by the lack of viral genetic material within eVLPs, we envisioned a strategy to encode the identity of each eVLP variant using barcoded single-guide RNAs (sgRNAs) within eVLP-packaged RNP cargos (Fig. 1a). In this scheme, each eVLP production vector expresses both an eVLP variant and a barcoded sgRNA that uniquely identifies that eVLP variant (Fig. 1a). These barcoded eVLP production vectors are introduced into producer cells under conditions that maximize the likelihood that each producer cell receives only a single barcoded vector and, therefore, produces only a single eVLP variant–barcoded sgRNA combination. This strategy generates barcoded eVLP libraries in which each unique eVLP variant packages sgRNAs containing a unique corresponding barcode (Fig. 1a).
a, Overview of the barcoded eVLP evolution system. Each unique eVLP variant is linked to a unique barcoded sgRNA on the same eVLP production vector. eVLP production maintains barcode–variant correspondence and yields a barcoded eVLP library. In the resulting library, each eVLP variant packages RNPs containing barcoded sgRNAs that encode the identity of that particular eVLP variant. Barcodes that are enriched following a selection for desired properties identify eVLP variants that possess the desired properties. b, Schematic of the mock production selection experiment with barcode 1 linked to a functional Gag–ABE construct and barcode 2 linked to a nonfunctional ABE only (no Gag) construct. c, Frequencies of barcodes 1 or 2 detected in either the producer-cell gDNA or the eVLP-packaged sgRNAs. Bars reflect the mean of n = 3 biological replicates and dots represent individual replicate values. d, Schematic of the mock transduction selection experiment with barcode 1 linked to wild-type VSV-G envelope protein and barcode 2 linked to an impaired VSV-Gmut envelope protein. e, Frequencies of barcodes 1 or 2 detected in either the eVLP-packaged sgRNAs or the delivered sgRNAs. Bars reflect the mean of n = 3 biological replicates and dots represent individual replicate values.
After subjecting a barcoded eVLP library to a selection for a desired property, eVLP variants surviving selection are identified by sequencing their sgRNAs and determining which barcodes are enriched in the postselection population compared to the input population (Fig. 1a). This scheme for evolving barcoded eVLPs in principle can be used to evolve different eVLP components—including capsid, envelope, cargo and other structural proteins—by placing the evolving component on the same vector as the barcoded sgRNA when constructing the library of eVLP production vectors (Fig. 1a). Additionally, this scheme is compatible with a wide range of selections for desired properties, including improved particle production, particle stability or transduction of a particular cell type in vitro or in vivo.
We first validated that barcoded sgRNAs are compatible with functional eVLP production. We inserted a 15-bp barcode sequence into the tetraloop of the sgRNA scaffold (Extended Data Fig. 1a), as previous studies showed that this location and length of insertion does not disrupt sgRNA function49,50. For all validation experiments, we used our previously developed v4 base editor (BE)-eVLPs16 that package a highly active adenine base editor (ABE8e) RNP cargo51. Standard v4 BE-eVLPs are produced by cotransfecting four expression plasmids into producer cells (Extended Data Fig. 1b), encoding the expression of (1) the Gag–ABE fusion; (2) the sgRNA that directs on-target base editing in the transduced cells; (3) the Moloney murine leukemia virus (MMLV) Gag–Pro–Pol polyprotein, which contains the required viral protease and other structural components; and (4) the vesicular stomatitis virus G (VSV-G) envelope protein.
We produced v4 eVLPs containing canonical or tetraloop-barcoded sgRNAs with four arbitrarily chosen barcodes and compared their potencies by measuring base editing efficiencies at the BCL11A enhancer locus in eVLP-transduced HEK293T cells. We observed that barcoded eVLPs exhibited comparable potency to standard eVLPs and that eVLPs produced with distinct barcoded sgRNAs exhibited comparable potencies (Extended Data Fig. 1c,d). Because the evolution scheme requires that the barcoded sgRNA and evolving eVLP component are expressed from the same vector, we also confirmed that a single vector containing both an sgRNA expression cassette and a Gag–ABE fusion could support efficient eVLP production and cargo delivery (Extended Data Fig. 1c). Reverse transcription quantitative PCR (RT–qPCR) analysis confirmed that eVLPs lacking Gag–ABE package 216-fold fewer sgRNA molecules compared to canonical v4 eVLPs, suggesting that sgRNA packaging in the absence of Gag–ABE is negligible and, therefore, not likely to influence selection outcomes (Extended Data Fig. 1e). These results together indicate that barcoded BE-eVLPs can be produced in a manner that preserves standard BE-eVLP properties.
Lastly, we validated that barcoded eVLPs can be used to distinguish between eVLP variants with different functional properties. To do so, we performed two mock selections: a selection for cargo-loaded eVLP production and a selection for eVLP transduction of HEK293T cells. We performed the mock eVLP production selection using two different BE-eVLP cargo constructs: (1) a standard Gag–ABE cargo construct used in v4 eVLPs and (2) a nonfunctional cargo construct containing an ABE but no Gag fusion, which almost completely abolishes ABE cargo loading into eVLPs16. We paired each of these two cargo constructs with a unique barcoded sgRNA and used lentiviral integration to generate producer cells expressing either barcode 1 (corresponding to Gag–ABE) or barcode 2 (corresponding to ABE only) (Fig. 1b). We then initiated eVLP production from a 1:1 mixture of these producer cells. Because only the barcode 1 (Gag–ABE) producer cells and not barcode 2 (ABE only) producer cells can produce functional eVLPs containing substantial amounts of ABE RNP cargo, we anticipated that barcode 1 would be enriched in eVLPs, while barcode 2 would be depleted (Fig. 1b). Indeed, we observed that barcode 1 was strongly enriched 13-fold (93% of sequencing reads) compared to barcode 2 (7% of sequencing reads) in eVLP-packaged sgRNAs even though barcodes 1 and 2 were equally represented in the original producer cell mixture (Fig. 1c).
Next, we performed a mock eVLP transduction selection using two different eVLP envelope constructs: (1) a standard VSV-G envelope construct that enables transduction of most cell types, and (2) an impaired VSV-Gmut envelope construct that reduces but does not completely eliminate the ability of viral particles to engage target cell surface receptors and deliver their packaged cargo in the absence of additional targeting ligands34,52,53. We produced VSV-G-pseudotyped eVLPs packaging barcode 1 and VSV-Gmut-pseudotyped eVLPs packaging barcode 2 and then transduced HEK293T cells with a 1:1 mixture of these barcoded eVLPs (Fig. 1d). Because VSV-G-pseudotyped eVLPs should more efficiently transduce cells and deliver RNP cargo compared to VSV-Gmut-pseudotyped eVLPs, we anticipated that barcode 1 would be enriched in sgRNAs retrieved from eVLP-transduced cells compared to sgRNAs packaged in the input eVLP mixture while barcode 2 would be depleted (Fig. 1d). Indeed, we observed that barcode 1 was enriched 2.4-fold (71% of sequencing reads) compared to barcode 2 (29% of sequencing reads) in sgRNAs retrieved from eVLP-transduced cells even though barcodes 1 and 2 were equally represented in the original eVLP mixture (Fig. 1e).
These results of the mock eVLP production selection and mock eVLP transduction selection demonstrate that barcoded sgRNAs can be used to label different eVLP variants and that barcodes that are enriched following a selection identify variants with increased fitness. Collectively, these findings validate key aspects of the barcoded eVLP evolution system and establish a framework for using barcoded sgRNAs to identify eVLP variants with desired properties.
Mutating and selecting a barcoded eVLP capsid library
Next, we applied the barcoded eVLP evolution system to mutate and select eVLP capsids with improved properties. The capsid proteins that are used in v4 eVLPs are identical to the capsid proteins used in wild-type MMLV, which have evolved in nature to be optimal for packaging viral genomes54,55. Therefore, wild-type MMLV capsids are likely not optimal for packaging large, non-native protein cargos such as ABEs in eVLPs. We hypothesized that remodeling the internal eVLP capsid surface to optimize ABE RNP cargo packaging instead of viral genome packaging could substantially improve eVLP properties, including potency per particle, number of cargo molecules packaged per particle, overall particle yield or titer and particle stability.
To mutate and select eVLP capsids to become more optimal for packaging ABE RNP cargo, we first designed and constructed a barcoded eVLP capsid library. This library contained 3,762 single-residue mutants of the MMLV Gag protein capsid (amino acids 215–313 and 413–479) and nucleocapsid (amino acids 480–513) domains in the Gag–ABE cargo construct (Extended Data Fig. 2a). We implemented a library construction strategy that preserved the association between barcodes and mutants to enable decoding of selection outcomes (Extended Data Fig. 2b,c). Barcodes were chosen such that no two barcode sequences were within four mismatches of each other to minimize the likelihood of incorrect barcode classification because of sequencing errors during barcode retrieval or mutations during eVLP production.
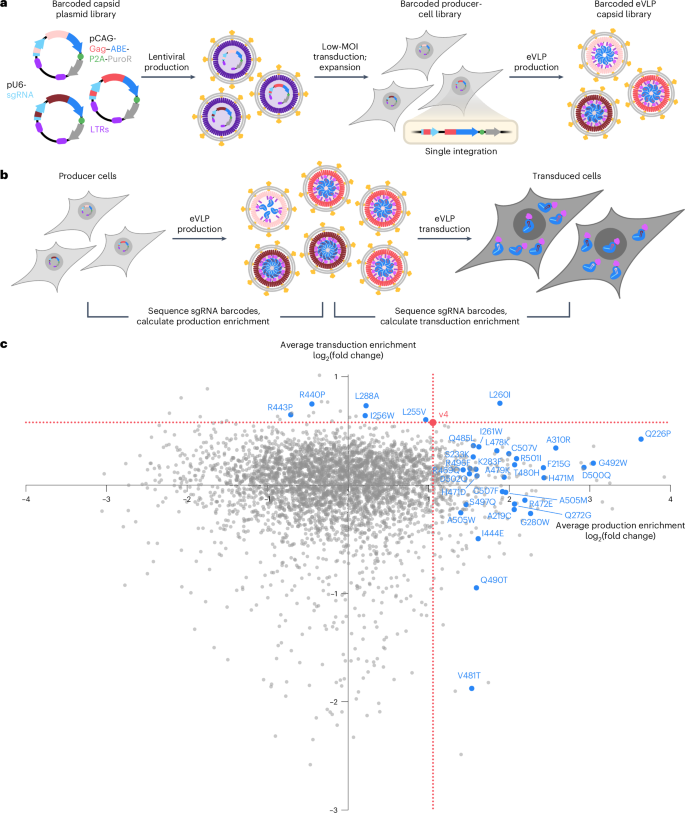
We used this barcoded plasmid library to generate a library of barcoded eVLP producer cells (Fig. 2a). Lentiviral transduction of producer cells at a low multiplicity of infection (MOI) followed by expansion of the transduced cells maximized the fraction of producer cells that each received a single barcode–capsid variant pair (Supplementary Table 5). High-throughput sequencing analysis of genomic DNA (gDNA) isolated from the expanded producer cell library revealed that 99% of all barcode sequences were detected (Extended Data Fig. 3). These results demonstrate the successful generation of barcoded eVLP plasmid and producer cell libraries, laying the foundation for eVLP evolution campaigns.
a, Schematic of the barcoded eVLP capsid library generation. Each unique capsid mutant was linked to a unique barcoded sgRNA on the same plasmid vector. These barcoded vectors were used to produce lentivirus, which was then used to generate a barcoded producer cell library in which each producer cell contained a single integration of a barcoded sgRNA and capsid mutant expression cassette. Following expansion of transduced cells, the barcoded producer cell library was transfected with the other plasmids necessary for eVLP production to generate a barcoded eVLP capsid library. b, Overview of selections for improved eVLP production and improved eVLP transduction. Barcodes enriched in eVLP-packaged sgRNAs relative to producer-cell gDNA identify capsid mutants that support improved eVLP production. Barcodes enriched in eVLP-transduced cells relative to eVLP-packaged sgRNAs identify capsid mutants that support improved eVLP transduction. c, Average barcode enrichment values for each capsid mutant in the production selection and transduction selection. Each capsid mutant is shown as a single dot whose x coordinate reflects the average production enrichment of the capsid mutant and y coordinate reflects the average transduction enrichment of the capsid mutant. The canonical capsid used in v4 eVLPs is shown as a red dot and the corresponding enrichment values associated with this dot are shown as dotted red lines. Capsid mutants selected for further characterization are shown as blue dots. Production and transduction enrichment values were calculated as the average of n = 2 replicates. Further details are provided in Extended Data Figs. 4 and 5 and Supplementary Table 3.
Selections reveal how capsid mutants affect eVLP properties
We subjected the barcoded eVLP capsid library to two separate selections (Fig. 2b): (1) a selection for improved eVLP production from producer cells, and (2) a selection for improved eVLP transduction of HEK293T cells. To perform a selection for improved eVLP production, we initiated eVLP production from the barcoded producer cell library (Methods). We purified the resulting library of barcoded eVLP capsid variants, isolated the eVLP-packaged sgRNAs and sequenced the barcodes that were present after this production selection. For each barcode sequence in the library, we calculated the eVLP production enrichment by comparing the frequency of that barcode in eVLP-packaged sgRNAs to the frequency of that barcode in the producer-cell gDNA. In this production selection, barcodes that display greater enrichment than the canonical eVLP capsid barcode identify candidate capsid mutants that support improved production compared to the canonical capsid (Extended Data Fig. 4a). Enriched barcodes, for example, might indicate that those capsid mutants package more RNP cargo molecules per particle than the canonical capsid or are produced at a higher titer, either of which could explain why those particular sgRNAs were more abundant in the produced eVLPs relative to producer-cell gDNA.
Approximately 8% of all capsid mutants in the library exhibited an average production enrichment higher than that of the canonical eVLP capsid (Fig. 2c and Extended Data Fig. 4b). Because the complete MMLV capsid consists of a complex assembly of thousands of capsid subunits54, it is likely that the majority of capsid mutations disrupt the carefully orchestrated process of capsid assembly, explaining the rarity of mutants enriched beyond that of the canonical eVLP capsid in the eVLP production selection. The enrichment of a subpopulation of capsid mutants in the production selection beyond canonical eVLPs (Fig. 2c and Extended Data Fig. 4b), however, supported our hypothesis that the wild-type MMLV capsid is not optimal for RNP cargo packaging and that eVLP capsids can be mutated and selected in the laboratory to improve this property.
In addition to improving eVLP production, the eVLP evolution system can be used to improve transduction of eVLPs into target cells (Fig. 2b). We incubated HEK293T cells with the purified barcoded eVLP capsid library and isolated sgRNAs that were successfully transduced into target cells after 6 h. For each barcode sequence in the library, we calculated the eVLP transduction enrichment by comparing the frequency of that barcode in the transduced HEK293T cells to the frequency of that barcode in the eVLP-packaged sgRNAs before incubation with HEK293T cells. Barcodes that are enriched to a higher degree than the canonical v4 eVLP barcode identify capsid mutants that support improved transduction relative to the v4 eVLP capsid (Extended Data Fig. 5a). Enriched barcodes, for example, might reflect capsid mutants that transduce target cells more efficiently because they are more stable or enter target cells more efficiently. Notably, we observed that only 0.7% of all capsid mutants in the library exhibited an average transduction enrichment greater than that of the canonical v4 eVLP capsid (Fig. 2c and Extended Data Fig. 5b). These findings support a model in which capsid mutants are more likely to improve eVLP production or RNP cargo packaging but rarely improve particle stability, cell entry or other characteristics that influence transduction.
By integrating the results from both the production and the transduction selections, we generated a landscape that reveals how each capsid mutant influences these two properties of eVLPs (Fig. 2c). The vast majority of capsid mutants exhibited worse production and transduction efficiencies compared to the canonical v4 eVLP capsid. While a handful of mutants showed selection enrichments that suggest improvements in either production or transduction, virtually no mutants exhibited improvements in both properties, suggesting that eVLP production and transduction efficiencies are dictated by distinct and potentially competing mechanisms. Certain clusters of mutations consistently impacted eVLP production and transduction. For example, R440P or R443P improved transduction but negatively impacted production (Fig. 2c). Conversely, L478K, A479K or T480H improved production but modestly impaired transduction (Fig. 2c). These observations suggest that remodeling the internal charged surfaces of the eVLP capsid is a potential strategy for optimizing eVLP capsids to better package and deliver RNP cargo. Together, the results of the eVLP capsid selections demonstrate the utility of the barcoded eVLP system, reveal new insights into how different capsid mutations influence eVLP properties and nominate potentially improved capsid mutants that warrant further characterization.
Combinations of capsid mutations improve eVLP potency
On the basis of the results of the production and transduction selections, we identified a set of 36 capsid mutants for further characterization (blue dots in Fig. 2c). We chose these mutants on the basis of their positive enrichments in both replicates of the production or transduction selections, prioritizing mutants that improved one property without substantially impairing the other property (Fig. 2c). To perform a high-throughput assessment of the potency of multiple different variants simultaneously, we produced different eVLP variants through transient transfection of eVLP plasmids into producer cells in different wells of 96-well plates, transduced HEK293T cells with the same volume of each eVLP variant at a subsaturating dose (Methods) and determined each the potency of each variant by measuring adenine base editing efficiencies at the sgRNA-specified target BCL11A enhancer locus in the transduced cells. These eVLP production conditions impose an eVLP stoichiometry of 25:75 Gag–ABE:Gag–Pro–Pol, a stoichiometry that we previously determined to be optimal for eVLP potency16. This 25:75 stoichiometry likely differs from the stoichiometry imposed during the barcoded library selection conditions because eVLP production from singly integrated Gag–ABE producer cell lines likely results in a low Gag–ABE:Gag–Pro–Pol ratio. While we anticipated that this stoichiometry difference might lead to differences between an individual mutant’s performance in selections versus in potency assays, we chose to compare all mutants to canonical v4 eVLPs using optimal transfection-based production conditions to assess whether any of these mutants could outperform v4 eVLPs in this most relevant setting.
We began by introducing each of the 36 capsid mutants into the v4 Gag–ABE construct and used canonical versions of the other components of the v4 eVLP architecture (wild-type MMLV Gag–Pro–Pol, VSV-G and standard sgRNA) to produce the evolved eVLP variants. In this context, we observed that most of the mutations did not improve eVLP potency compared to v4 eVLPs (Extended Data Fig. 6a and Supplementary Fig. 1a), indicating that incorporating the capsid mutations into the Gag–ABE construct alone was not sufficient to improve potency.
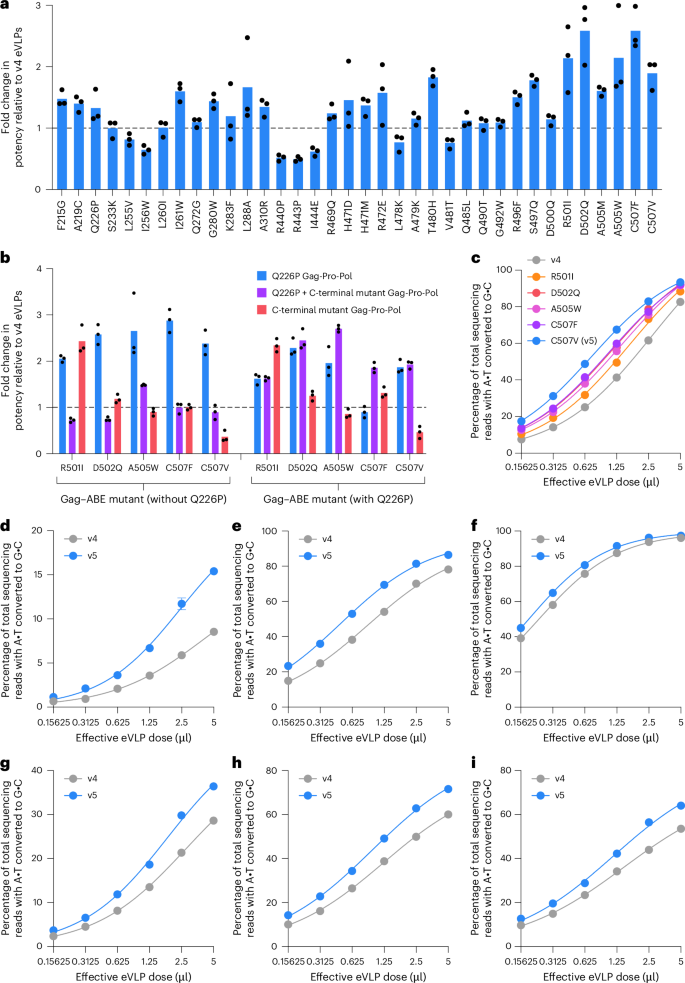
Because the processed Gag protein expressed in the Gag–Pro–Pol construct, along with the processed Gag protein expressed in the Gag–ABE construct, are both important contributors to the overall eVLP capsid, we also incorporated the capsid mutations into the Gag–Pro–Pol construct used for eVLP production (Extended Data Fig. 6b). We first incorporated the Q226P mutation into the Gag–Pro–Pol construct (hereafter referred to as GagQ226P–Pro–Pol), because the Q226P mutation was the most strongly enriched mutation from the production selection that only modestly impaired transduction (Fig. 2c). Next, we assessed the potency of the same 36 capsid mutants in the Gag–ABE construct but now using the GagQ226P–Pro–Pol instead of the wild-type MMLV Gag–Pro–Pol. In this context, many of the tested capsid mutants exhibited 2–3-fold increases in BE delivery potency compared to v4 eVLPs (Fig. 3a and Supplementary Fig. 1b). In general, we observed that mutants that modestly improved production exhibited greater improvements in potency compared to mutants that substantially improved production (Extended Data Fig. 7), consistent with a model in which excessive improvements in eVLP production or cargo packaging might be detrimental to overall eVLP potency.
a, Fold change in eVLP potency compared to v4 eVLPs of each capsid mutant incorporated individually into the Gag–ABE construct and paired with GagQ226P–Pro–Pol. b, Fold change in eVLP potency relative to v4 eVLPs of each C-terminal capsid mutant with or without the Q226P mutant incorporated into either the Gag–ABE only, Gag–Pro–Pol only or both Gag–ABE and Gag–Pro–Pol. In a,b, bars reflect the mean of n = 3 biological replicates and dots represent individual replicate values. c, Comparison of v4 eVLPs and Gag–ABE mutants paired with the GagQ226P–Pro–Pol across a range of eVLP doses. Adenine base editing efficiencies at position A7 of the BCL11A enhancer site in HEK293T cells are shown. eVLPs were produced at a concentration of approximately 5 × 108 eVLPs per microlitre. d–i, Comparison of v4 and v5 eVLPs in mouse N2A cells across a range of eVLP doses. Adenine base editing efficiencies are shown at position A8 of the Angptl3 exon 7 splice acceptor site (d), position A4 of the Rosa26 site (e), position A9 of the Dnmt1 site (f), position A6 of the Pcsk9 exon 4 splice acceptor site (g), position A4 of the Pcsk9 exon 6 splice donor site (h) and position A8 of the Pcsk9 exon 8 splice acceptor site (i). In c–i, dots and error bars represent the mean ± s.e.m. of n = 3 biological replicates. Data were fit to four-parameter logistic curves using nonlinear regression.
In light of the discovery that different Gag–ABE and Gag–Pro–Pol capsid mutants can synergize, we systematically evaluated the effects of incorporating different combinations of mutations into the Gag–ABE or Gag–Pro–Pol constructs. We selected five Gag–ABE mutants that exhibited the highest potency when paired with the GagQ226P–Pro–Pol: R501I, D502Q, A505W, C507F and C507V (Fig. 3a). We tested all possible combinations of each C-terminal mutant and Q226P mutant incorporated into the Gag–ABE only, Gag–Pro–Pol only or both Gag–ABE and Gag–Pro–Pol (Fig. 3b). Notably, four of the five C-terminal Gag–ABE mutants still performed best when paired with the GagQ226P–Pro–Pol instead of a matched Gag–Pro–Pol containing that same C-terminal mutant (Fig. 3b). These findings reveal the complex interplay between different capsid mutations and underscore the importance of assessing these mutations in several possible eVLP configurations.
We next evaluated the potency of these five eVLP variants (R501I, D502Q, A505W, C507F or C507V Gag–ABE mutants paired with GagQ226P–Pro–Pol) compared to v4 eVLPs across a range of doses in HEK293T cells (Fig. 3c). As expected, all eVLP variants exhibited improved base editing efficiencies at all doses tested compared to v4 eVLPs (Fig. 3c). In particular, the GagC507V–ABE + GagQ226P–Pro–Pol combination exhibited an average overall 3.7-fold improvement in potency (half-maximal effective concentration, EC50) (Fig. 3c). This substantial improvement in potency is comparable to what we observed between v2 and v1 eVLPs or v3 and v2 eVLPs in our previous study16. Therefore, we designated the GagC507V–ABE + GagQ226P–Pro–Pol combination as v5 BE-eVLPs (Fig. 3c).
We also investigated whether incorporating mutations identified in the BE-eVLP selections might also improve the delivery potency of eVLPs that package other gene editing cargos, such as Cas9 nuclease or PEs. We observed that Cas9-eVLPs containing GagC507V–Cas9 + GagQ226P–Pro–Pol exhibited an average twofold improvement in potency (EC50 value) compared to v4 Cas9-eVLPs at both the BCL11A enhancer site and the EMX1 site in HEK293T cells (Extended Data Fig. 8a,b). Therefore, we designated the GagC507V–Cas9 + GagQ226P–Pro–Pol combination as v5 Cas9-eVLPs. We also observed that v3 or v3b PE2-eVLPs36 containing the Q226P mutation incorporated into the relevant Gag–Pro–Pol constructs exhibited comparable potency to canonical v3 or v3b PE2-eVLPs (Extended Data Fig. 8c,d). Together, these results suggest that mutations identified in the BE-eVLP selections can similarly improve the delivery potency of Cas9-eVLPs, which are highly similar in architecture to BE-eVLPs, but not PE-eVLPs, which contain multiple additional structural components and RNA-binding proteins specific to PE cargos that might behave differently when combined with the evolved capsid mutants. These findings also raise the possibility that PE-eVLPs or other eVLPs with distinct architectures might benefit from cargo-specific or architecture-specific production and transduction selections that are analogous to those we performed using BE-eVLPs in this study.
Lastly, we evaluated the potency of v5 BE-eVLPs compared to v4 BE-eVLPs across a range of doses and target genomic loci in mouse Neuro-2a (N2A) cells (Fig. 3d–i). In all tested cases, v5 BE-eVLPs exhibited improved potency compared to v4 BE-eVLPs. In general, the base editing efficiency at a given dose of v4 BE-eVLPs can be achieved using a 2–4-fold lower dose of v5 BE-eVLPs (Fig. 3c–i). We anticipate that v5 eVLPs will be especially useful for eVLP delivery applications that are limited by the maximum administrable dose. Collectively, these results demonstrate that the barcoded eVLP evolution system successfully generated improved eVLP capsid mutants that enabled the discovery of v5 BE-eVLPs with improved delivery potency compared to previous-best v4 BE-eVLPs.
v5 eVLPs improve base editing potency in primary human HSPCs
To further investigate the potential utility of v5 eVLPs, we compared the potencies of v4 and v5 BE-eVLPs in primary human hematopoietic stem and progenitor cells (HSPCs). Ex vivo gene editing of autologous HSPCs followed by transplantation has proven to be powerful approach for treating blood disorders, including sickle cell disease and β-thalassemia56,57,58,59,60,61. Nuclease-mediated disruption of the BCL11A erythroid-specific enhancer in HSPCs leads to the induction of fetal hemoglobin expression in erythrocytes, which is sufficient to rescue disease phenotypes associated with these blood disorders and is the first US Food and Drug Administration-approved gene editing drug56,61,62. Therapeutic induction of fetal hemoglobin has also been achieved using ex vivo cytosine or adenine base editing to install precise single-base conversions within the BCL11A erythroid-specific enhancer or the fetal hemoglobin (HBG) promoter in HSPCs57,63,64. Because base editing avoids negative consequences associated with nuclease-generated DNA double-strand breaks and uncontrolled mixtures of indel products57, base editing-mediated disruption of the BCL11A erythroid-specific enhancer in HSPCs could potentially offer advantages over nuclease-mediated disruption.
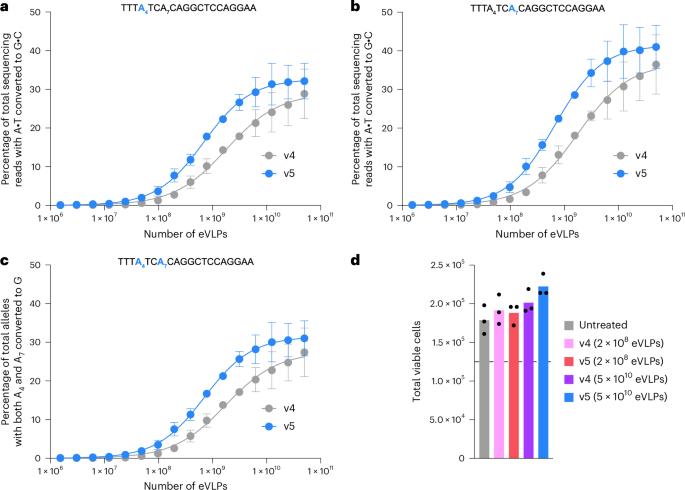
To evaluate eVLP-mediated base editing in HSPCs, we transduced healthy human donor HSPCs (Methods) with v4 or v5 BE-eVLPs packaging ABE8e and an sgRNA targeting the BCL11A erythroid-specific enhancer locus. We observed substantially higher target adenine base editing efficiencies from v5 BE-eVLPs compared to v4 BE-eVLPs across all doses tested (Fig. 4a–c and Extended Data Fig. 9) Overall, v5 BE-eVLPs exhibited an average 2.6-fold improvement in potency (EC50 value) in primary human HSPCs compared to v4 BE-eVLPs (P < 0.0001, extra sum-of-squares F-test) (Fig. 4b). Notably, the maximum editing efficiency achieved with the highest tested dose of v4 BE-eVLPs was achieved with a 16-fold lower dose of v5 BE-eVLPs (Fig. 4b). These results suggest that v5 eVLPs could greatly simplify the application of eVLPs in large-scale studies by minimizing the dose required to achieve efficient editing, thereby substantially reducing the necessary manufacturing burden. The total number of viable HSPCs detected 48 h after treatment with v4 or v5 eVLPs was comparable to that of untreated HSPC controls, indicating that eVLPs do not substantially perturb HSPC viability (Fig. 4d). Collectively, these results further demonstrate the utility of v5 eVLPs by revealing their improved performance compared to v4 eVLPs in a therapeutically relevant primary human cell type.
a–c, Comparison of v4 and v5 BE-eVLPs in primary human HSPCs across a range of eVLP doses: adenine base editing efficiencies at position A4 of the BCL11A enhancer site (a), position A7 of the BCL11A enhancer site (b) and both positions A4 and A7 of the BCL11A enhancer site (c). In a–c, dots and error bars represent the mean ± s.e.m. of n = 3 biological replicates. Data were fit to four-parameter logistic curves using nonlinear regression. The number of eVLPs was quantified using anti-MLV p30 ELISAs (Methods). Plots showing individual replicate values are provided in Extended Data Fig. 9. d, Total viable cell counts measured 48 h after eVLP transduction. The dotted line denotes the initial number of cells measured in each condition (1.25 × 105 cells) immediately before eVLP transduction. Bars reflect the mean of n = 3 biological replicates and dots represent individual replicate values.
v5 eVLPs exhibit improved cargo packaging and release
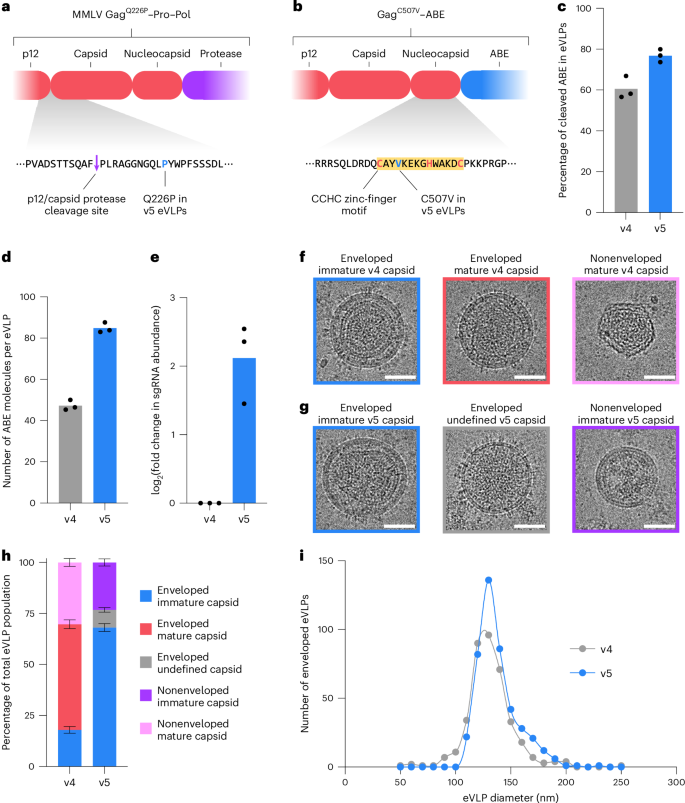
Next, we sought to illuminate the effects of the capsid mutations in v5 eVLPs. The Q226P mutation is located at the N terminus of the capsid domain of Gag, directly upstream of the internal protease cleavage site that separates the capsid and p12 domains following particle maturation (Fig. 5a). Because of its proximity to this protease cleavage site, it is possible that the Q226P mutation alters the rate of cleavage at this site, which could impact capsid formation kinetics to improve packaging large RNP cargos. By contrast, the C507V mutation is located near the C terminus of the nucleocapsid domain of Gag (Fig. 5b). The C507V mutation disrupts the second cysteine in the CCHC zinc-finger motif within the nucleocapsid domain (Fig. 5b) that is known to be required for packaging and replicating viral genomes in wild-type MMLV65,66,67. Because eVLPs lack viral genomes, this CCHC zinc-finger motif is likely no longer required in eVLPs and is instead free to be mutated during selection for improved RNP cargo packaging. The barcoded eVLP evolution system, therefore, identified a capsid mutation that removes a native viral function not used in RNP-delivering eVLPs, further highlighting the benefits of mutating and selecting eVLP capsids to become more optimal for packaging non-native RNP cargos instead of viral genomes.
a, Schematic of the Q226P mutant within the GagQ226P–Pro–Pol in v5 eVLPs, located directly downstream of the internal p12–capsid protease cleavage site. b, Schematic of the C507V mutant within the GagC507V–ABE in v5 eVLPs, located within the CCHC zinc-finger motif in the nucleocapsid domain. c, Percentage of cleaved ABE cargo detected in v4 or v5 eVLPs. d, Quantification of ABE molecules per eVLP by anti-Cas9 and anti-MLV (p30) ELISA (Methods). e, Fold change in eVLP-packaged sgRNA abundance measured by RT–qPCR using sgRNA-specific primers, normalized relative to sgRNA abundance in v4 eVLPs. c–e, Bars reflect the mean of n = 3 replicates and dots represent individual replicate values. f, Representative cryo-EM images of v4 eVLPs. g, Representative cryo-EM images of v5 eVLPs. Scale bars, 50 nm. h, Classification and quantification of the types of eVLP capsids observed in the cryo-EM images of v4 or v5 eVLPs. Bars reflect the mean ± s.e.m. of sample proportion for n = 551 total v4 eVLPs or n = 577 total v5 eVLPs. i, Quantification of the particle diameter of every enveloped v4 (n = 384) or enveloped v5 eVLP (n = 438) observed in the cryo-EM images. Discrete histograms of particle counts were interpolated with an Akira spline curve.
To experimentally characterize the effects of the v5 capsid mutations, we analyzed the protein and sgRNA content of v4 and v5 eVLPs. We previously identified efficient cargo release as a key determinant of eVLP potency16. Western blot analysis of lysed eVLPs revealed more efficient cleavage of the capsid–cargo linker in v5 eVLPs compared to v4 eVLPs (Fig. 5c and Extended Data Fig. 10a), indicating that improved cargo release in v5 eVLPs likely contributes to their improved potency. Next, we quantified the number of ABE protein molecules packaged per eVLP by ELISA and observed a 1.8-fold increase in protein packaging in v5 eVLPs compared to v4 eVLPs (Fig. 5d). We also detected a 4.3-fold increase in the sgRNA packaging levels by RT–qPCR in v5 eVLPs compared to v4 eVLPs (Fig. 5e). The combined increases in protein and sgRNA packaging suggest that v5 eVLPs package substantially more active RNPs per particle compared to v4 eVLPs, which likely contributes to their improved potency.
Our previous attempts to improve cargo packaging beyond that of v4 eVLPs resulted in increased protein packaging but not sgRNA packaging16. It is, therefore, noteworthy that the v5 capsid mutations evolved to improve RNP packaging and not just protein packaging, likely because barcoded sgRNA abundance was used as the readout for all selections and, thus, the selection system rewarded higher sgRNA packaging levels. We observed that v5 eVLPs are equally compatible with barcoded sgRNAs and standard sgRNAs, consistent with our hope that the activity of evolved eVLPs would not be strongly dependent on the use of barcoded sgRNAs (Extended Data Fig. 10b). Together, these results indicate that v5 eVLPs exhibit improved RNP cargo packaging and release compared to v4 eVLPs, suggesting a mechanistic hypothesis behind their improved potency.
v5 eVLPs exhibit altered capsid structure and particle sizes
We next sought to further characterize the physical and structural properties of v4 and v5 eVLPs. Previous studies investigated the internal structure of wild-type MMLV particles and observed that wild-type MMLV capsids exist in either an immature or mature state54,68. Immature capsids exist before proteolytic processing of Gag and form a single-layered spherical structure inside the viral envelope54. Mature capsids are generated after proteolytic processing of Gag and form a multilayered irregular polyhedral structure inside the viral envelope54. Mature capsids are generally required for wild-type retrovirus infection and successful nuclear import of the viral RNA genome in infected cells54,69. Previous studies have used various high-resolution imaging approaches to readily distinguish the structures and characteristics of mature and immature MMLV capsids54,68.
We performed cryo-electron microscopy (cryo-EM) of purified v4 or v5 eVLPs and analyzed the resulting cryo-EM images to classify all observed eVLPs based on their internal capsid structures. Cryo-EM analysis of v4 eVLPs revealed the presence of three distinct structural classes: (1) enveloped immature v4 capsids; (2) enveloped mature v4 capsids; and (3) nonenveloped mature v4 capsids (Fig. 5f). Enveloped immature v4 capsids displayed the characteristic spherical capsid organization found in immature wild-type MMLV capsids (Fig. 5f), including a single thick striated layer that corresponds to the immature conformation of the N-terminal and C-terminal domains of the capsid54. By contrast, enveloped mature v4 capsids instead displayed an irregular polyhedral organization and lacked a thick striated layer (Fig. 5f), indicating a transition to the mature conformation of the capsid’s N-terminal and C-terminal domains54. Nonenveloped mature v4 capsids displayed a polyhedral shape and are likely not functional through a canonical VLP delivery mechanism because they lack the viral envelope and envelope proteins required for transducing target cells (Fig. 5f). Of all v4 eVLPs detected in the cryo-EM images, 52% contained enveloped mature capsids, 18% contained enveloped immature capsids and 30% contained nonenveloped mature capsids (Fig. 5h).
Cryo-EM analysis of v5 eVLPs revealed the presence of three distinct structural classes that were markedly different from those found in v4 eVLPs: (1) enveloped immature v5 capsids; (2) enveloped undefined v5 capsids; and (3) nonenveloped immature v5 capsids (Fig. 5g). Enveloped immature v5 capsids displayed the characteristic thick striated capsid layer found in immature v4 capsids and immature wild-type MMLV capsids but immature v5 capsids appeared less closely associated with the viral envelope compared to canonical immature capsids (Fig. 5g). Enveloped undefined v5 capsids displayed neither a thick striated immature capsid layer nor an irregular polyhedral mature capsid (Fig. 5g). Nonenveloped immature v5 capsids displayed a spherical shape and are likely not functional through a canonical VLP delivery mechanism because they lack the viral envelope and envelope proteins required for transducing target cells (Fig. 5g). Of all v5 eVLPs detected in the cryo-EM images, not a single v5 capsid with canonically mature morphology was observed (Fig. 5h). Instead, 68% of all v5 particles contained enveloped immature capsids, 8.7% contained enveloped undefined capsids and 23% contained nonenveloped immature capsids (Fig. 5h). These analyses reveal that the capsid mutations in v5 eVLPs substantially alter the capsid structure compared to v4 eVLPs and potentially inhibit capsid maturation.
The absence of mature capsids in v5 eVLPs indicates that, while mature capsids are required for infectious wild-type MMLV particles, mature capsids are not required for eVLP-mediated delivery of BE RNP cargos. Indeed, because v5 eVLPs are more potent than v4 eVLPs, it is possible that immature capsids are not only sufficient for eVLP delivery but are actually more optimal for eVLP delivery than mature capsids. These results are also consistent with a recent report that demonstrated that mature capsids are not required for successful delivery of Cas9 RNP cargos by human immunodeficiency virus-derived VLPs53. While the lack of mature v5 capsids suggests a lack of complete proteolytic cleavage at every internal site within GagQ226P–Pro–Pol, we demonstrated above that proteolytic cleavage of the capsid–cargo linker in GagC507V–ABE is still efficient in v5 eVLPs (Fig. 5c and Extended Data Fig. 10a), which ensures efficient RNP cargo release into the transduced cells. These findings provide additional evidence to support the hypothesis that the optimal capsids for RNP-packaging eVLPs are distinct from the canonical capsids in viral RNA-packaging retroviruses.
Lastly, we analyzed the cryo-EM images to characterize the size distribution of enveloped v4 and v5 eVLPs. We observed that v5 eVLPs were overall slightly larger in mean diameter (137 ± 0.87 nm) compared to v4 eVLPs (131 ± 1.06 nm) (Fig. 5i). Furthermore, the size distribution of v5 eVLPs was significantly skewed toward larger particle diameters compared to the size distribution of v4 eVLPs (P < 0.0001, Kolmogorov–Smirnov test) (Fig. 5i). The increased size of v5 eVLPs might enable them to accommodate more cargo molecules per particle compared to v4 eVLPs, consistent with our observation above (Fig. 5d). Collectively, these analyses further illuminate the effect of the capsid mutations on various eVLP properties and the differences between v4 and v5 eVLPs that may contribute to the improved potency of v5 eVLPs.