OBiS Insights pre-meeting ESMO24 report is based on a review of the 2,960 abstract titles of which 275 new not yet approved drugs have been profiled.
NEW YORK, NY, UNITED STATES, September 12, 2024 /EINPresswire.com/ — OBiS, a New York City-based healthcare analytics consulting firm, has developed a pre-meeting report based on the abstract titles from the upcoming European Society of Medical Oncology (ESMO) congress. The report is available free of charge via the following link:
OBiS Insights ESMO24 Pre-Meeting Report
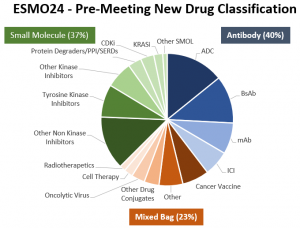
The OBiS Insights report consists of 275 “new”, not yet approved cancer drugs classified by modality as follows:
40% (109) Antibody-Based Drugs: Antibody-Drug Conjugates (39), Bispecific Antibodies (32), Other Monoclonal Antibodies (21), Immune Check Point Inhibitors (17).
37% (103) Small Molecule Drugs: Non-Kinase Inhibitors (36), Tyrosine Kinase Inhibitors (22), Other Kinase Inhibitors (18), Protein Degraders/PPI/SERDs (9), CDK Inhibitors (9), KRAS Inhibitors (6), Other Small Molecules (3).
23% (63) “Mixed Bag” Drugs: Cancer Vaccines (18), Other Mixed Bag (16), Other Drug Conjugates (10), Oncolytic Viruses (9), Cell Therapy (6), Radiotherapeutics (4).
IMPORTANT NOTE. The OBiS Insights pre-meeting report excludes drugs not specifically mentioned in the abstract titles. The report will be updated as the conference progresses. Additionally, some overlap between categories and exceptions is noted within the report.
The OBiS Insights report also includes a summary of key insights from the recent ASCO24 annual meeting and ASCO24BT meeting in Yokohama, Japan. Of the 275 drugs to be presented at ESMO24, 118 (43%) were previously discussed at these ASCO meetings.
Several cancer drugs approved in China but not yet in the United States or Europe (as of September 12, 2024)—such as cardunilimab, donafenib, flumatinib, penpulimab, pyrotinib, serplulimab, sintilimab, and vebreltinib—are excluded from this report but may be covered in a future update.
ABOUT OBiS
OBiS is a privately owned healthcare analytics firm based in New York City, specializing in business development, forecasting, new product planning, key opinion leader identification, market research, clinical trial recruitment analytics, and custom data services. OBiS serves biotechnology and pharmaceutical companies through the development of validated AI-driven analytics. Founded in 2002 by Rick Beasley, former Director of Oncology Market Research at Bristol-Myers Squibb, OBiS expanded in 2020 with a subsidiary office in Oloron-Sainte-Marie, France.
DISCLAIMER
The information contained in this press release is for EDUCATIONAL PURPOSES ONLY and is not intended to provide any medical or financial investment advice.
Richard Beasley
OBIS
+1 646-988-7679
email us here
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content “as is” without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
![]()